Shamees Aden has developed a concept for self-repairing running shoes made from 3D-printed synthetic biological material.


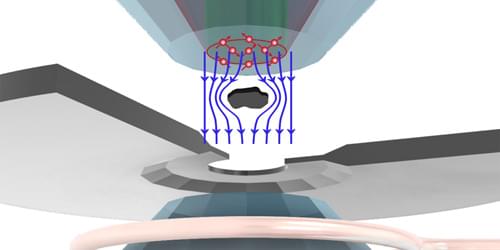
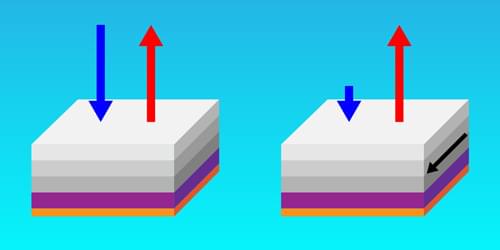
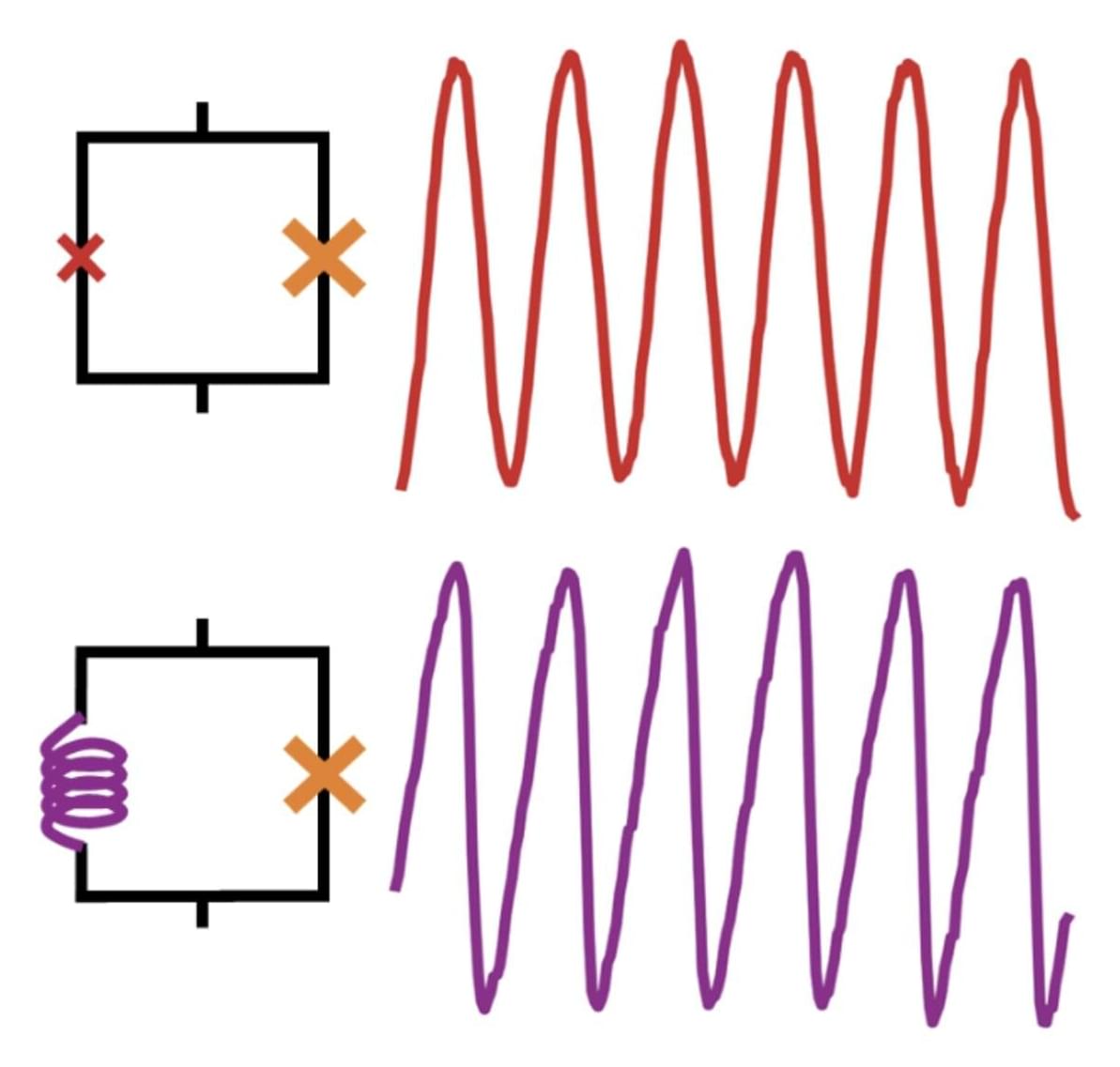
A newly designed structure exhibits the largest-recorded emissivity–absorptivity difference, a property that could prove useful in energy-harvesting and cloaking devices.
Hot objects glow. From the warmth of a stovetop to the invisible heat radiating from a building’s roof, thermal radiation flows outward. But it also flows inward in a reciprocal manner. This means that at thermal equilibrium, an object’s ability to thermally emit light in one direction, described as emissivity, is equal to its ability to absorb the same light coming in from the other direction, known as absorptivity. But what if this rule could be violated?
In a new study, Zhenong Zhang and colleagues from Pennsylvania State University demonstrate this exciting possibility [1]. The researchers apply an external magnetic field to a layered material, creating a system that breaks Lorentz reciprocity—a common symmetry that relates electromagnetic inputs and outputs. They then show that this nonreciprocal system exhibits much higher emissivity than absorptivity in the same direction. The observed difference between emissivity and absorptivity is twice that observed in previous experiments, thus setting a new benchmark in the field. These results pave the way for future technologies such as thermal diodes, radiative heat engines, and infrared camouflage.

For the first time, researchers have seen how light behaves during a mysterious phenomenon called ‘imaginary time’
When you shine light through almost any transparent material, the gridlock of electromagnetic fields that make up the atomic alleys and side streets will add a significant amount of time to each photon’s commute.
This delay can tell physicists a lot about how light scatters, revealing details about the matrix of material the photons must navigate. Yet until now, one trick up the theorist’s sleeve for measuring light’s journey – invoking imaginary time – has not been fully understood in practical terms.

Researchers at Northeastern University have discovered how to change the electronic state of matter on demand, a breakthrough that could make electronics 1,000 times faster and more efficient.
By switching from insulating to conducting and vice versa, the discovery creates the potential to replace silicon components in electronics with exponentially smaller and faster quantum materials.
“Processors work in gigahertz right now,” said Alberto de la Torre, assistant professor of physics and lead author of the research. “The speed of change that this would enable would allow you to go to terahertz.”
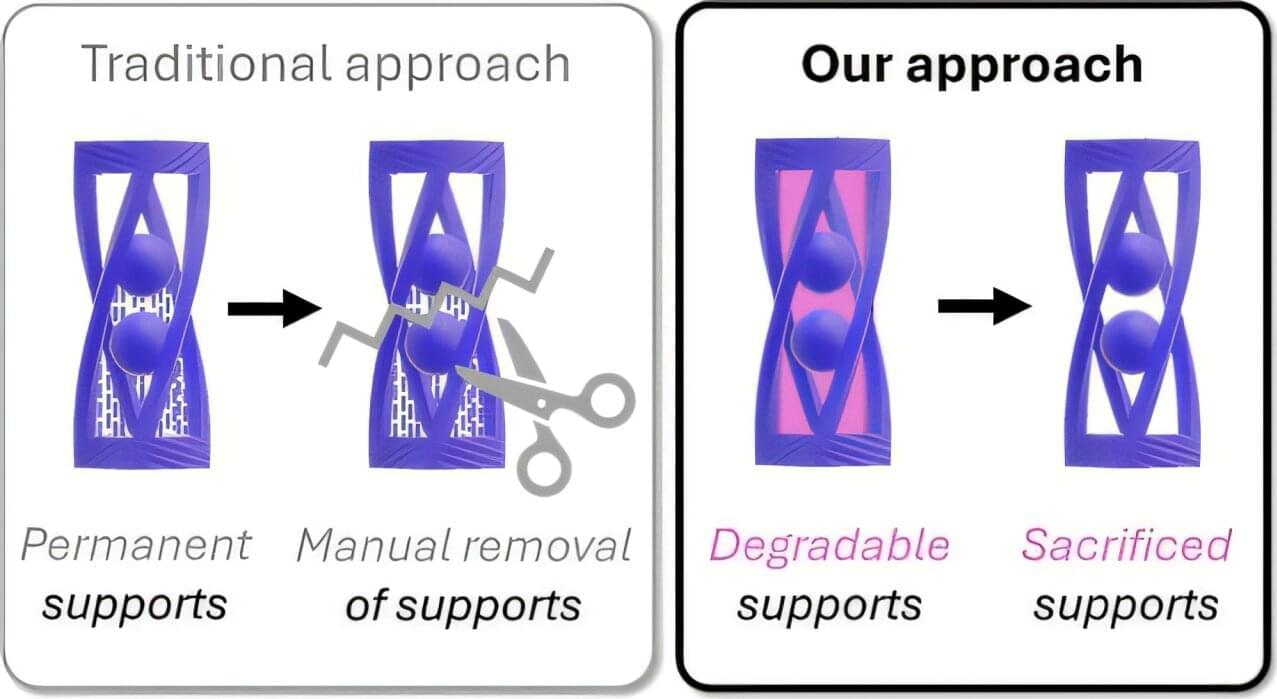
Lawrence Livermore National Laboratory (LLNL) researchers have developed a novel 3D printing technique that uses light to build complex structures, then cleanly dissolves the support material, expanding possibilities in multi-material additive manufacturing (AM).
In 3D printing, traditional supports often add time, waste and risk to the process, especially when printing intricate parts. But in a new study published in ACS Central Science, an LLNL team—in collaboration with University of California, Santa Barbara (UCSB) researchers—outlines a “one-pot” printing approach that uses two light wavelengths to simultaneously create permanent structures and temporary supports from a single resin formulation.
The method addresses a longstanding challenge in AM: how to fabricate suspended or overhanging features without cumbersome scaffolding requiring manual removal, which is a key hurdle to the widespread adoption of digital light processing (DLP) 3D printing technologies.
Superconductivity is an advantageous physical phenomenon observed in some materials, which entails an electrical resistance of zero below specific critical temperatures. This phenomenon is known to arise following the formation of so-called Cooper pairs (i.e., pairs of electrons).
There are two known types of superconductivity, known as conventional and unconventional superconductivity. In conventional superconductors, the formation of Cooper pairs is mediated by the interaction between electrons and phonons (i.e., vibrations in a crystal’s lattice), as explained by Bardeen-Cooper-Schrieffer (BCS) theory.
Unconventional superconductors, on the other hand, are materials that exhibit a superconductivity that is not prompted by electron–phonon interactions. While many past studies have tried to shed light on the mechanisms underpinning unconventional superconductivity, its underlying physics remains poorly understood.
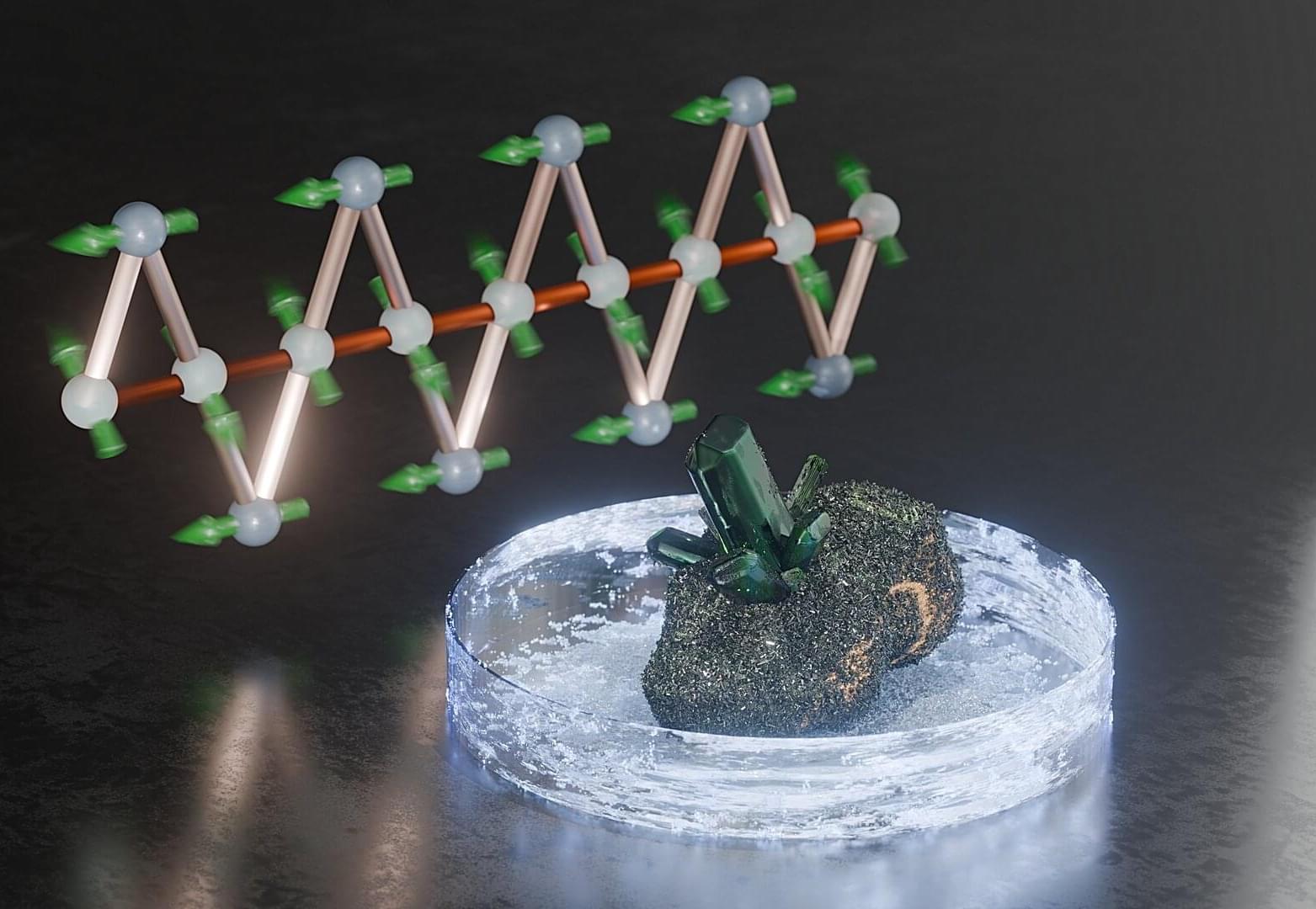
Natural crystals fascinate with their vibrant colors, their nearly flawless appearance and their manifold symmetrical forms. But researchers are interested in them for quite different reasons: Among the countless minerals already known, they always discover some materials with unusual magnetic properties.
One of these is atacamite, which exhibits magnetocaloric behavior at low temperatures—that is, the material’s temperature changes significantly when it is subjected to a magnetic field. A team headed by TU Braunschweig and the HZDR has now investigated this rare property. In the long term, the results, now published in Physical Review Letters, could help to develop new materials for energy-efficient magnetic cooling.
The emerald-green mineral atacamite, named for the place it was first found, the Atacama Desert in Chile, gets its characteristic coloring from the copper ions it contains. These ions also determine the material’s magnetic properties: they each have an unpaired electron whose spin gives the ion a magnetic moment —comparable to a tiny needle on a compass.
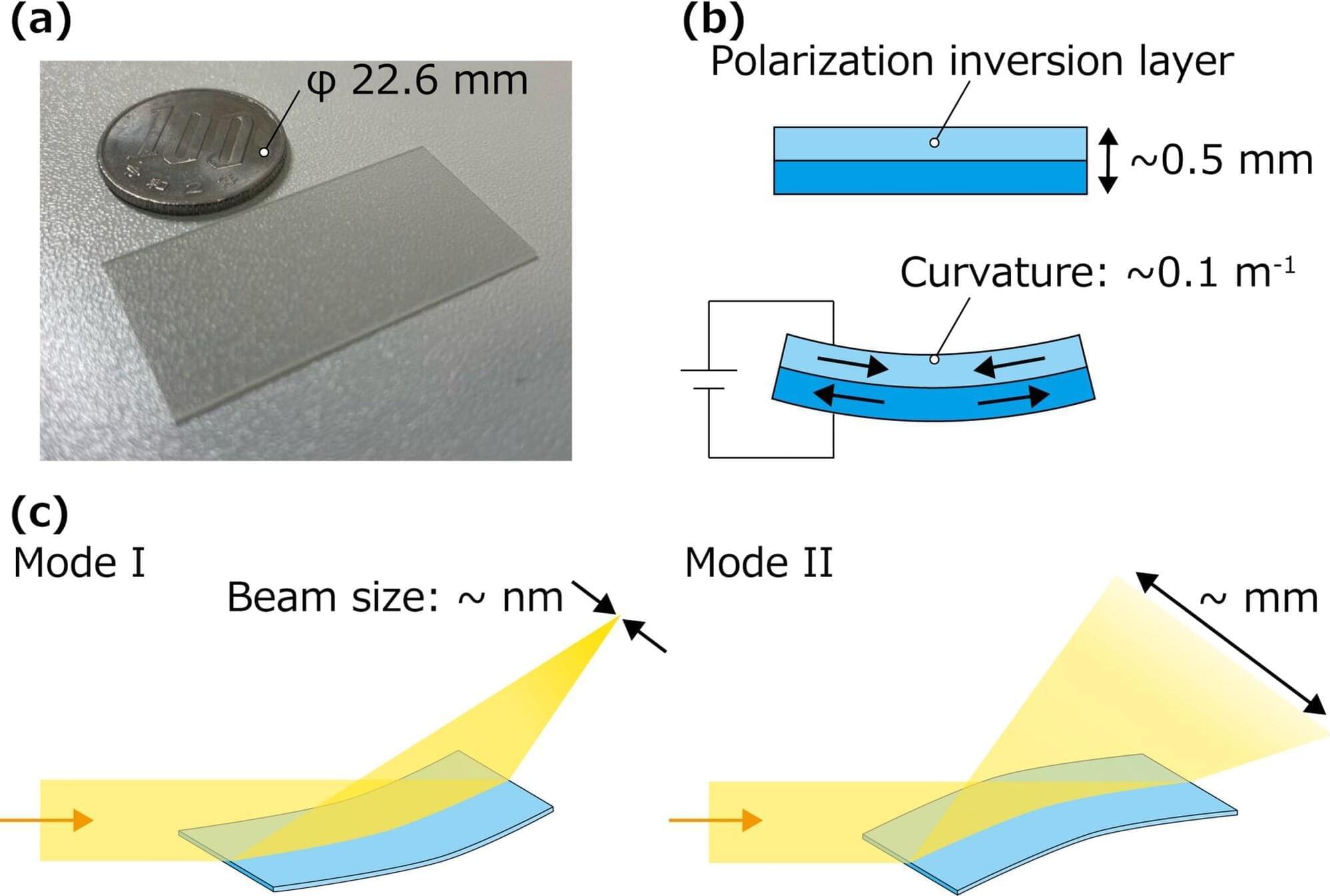
Using only a single-crystal piezoelectric thin wafer of lithium niobate (LN) instead of the usual two-part structure, a group from Nagoya University in Japan has created a deformable mirror that changes X-ray beam size by more than 3,400 times. This improved tuning range enhances both imaging and analysis, especially for the X-rays used in industry.
Their technique is based on LN, a material that has piezoelectricity, meaning that it changes its surface shape in response to voltage. Traditional X-ray mirrors are rigid and resistant to being deformed, making it difficult to adapt them to changing experimental conditions in real time, but the new technique can significantly change beam size, making it useful for a range of situations encountered in industry.
The study is published in the journal Scientific Reports.