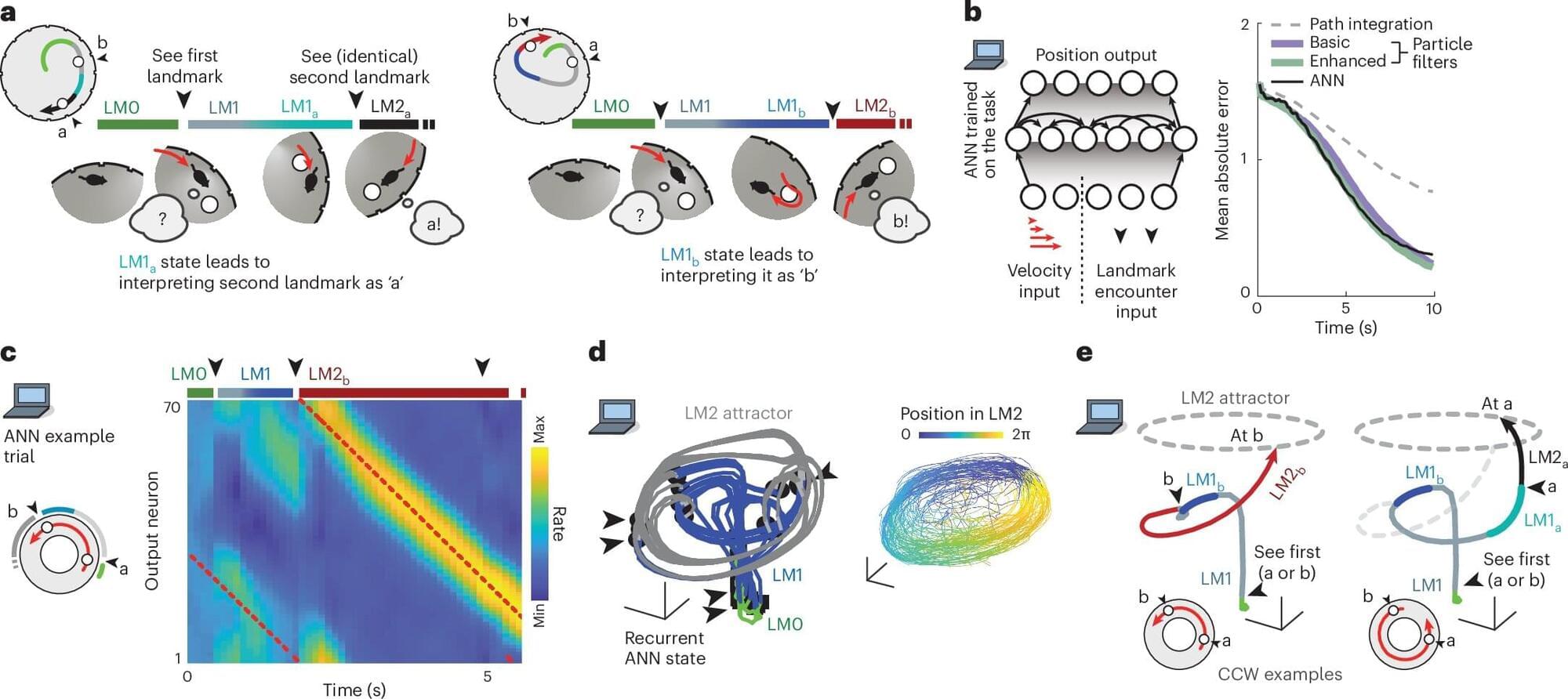
When navigating a place that we’re only somewhat familiar with, we often rely on unique landmarks to help make our way. However, if we’re looking for an office in a brick building, and there are many brick buildings along our route, we might use a rule like looking for the second building on a street, rather than relying on distinguishing the building itself.
Until that ambiguity is resolved, we must hold in mind that there are multiple possibilities (or hypotheses) for where we are in relation to our destination. In a study of mice, MIT neuroscientists have now discovered that these hypotheses are explicitly represented in the brain by distinct neural activity patterns.
This is the first time that neural activity patterns that encode simultaneous hypotheses have been seen in the brain. The researchers found that these representations, which were observed in the brain’s retrosplenial cortex (RSC), not only encode hypotheses but also could be used by the animals to choose the correct way to go.