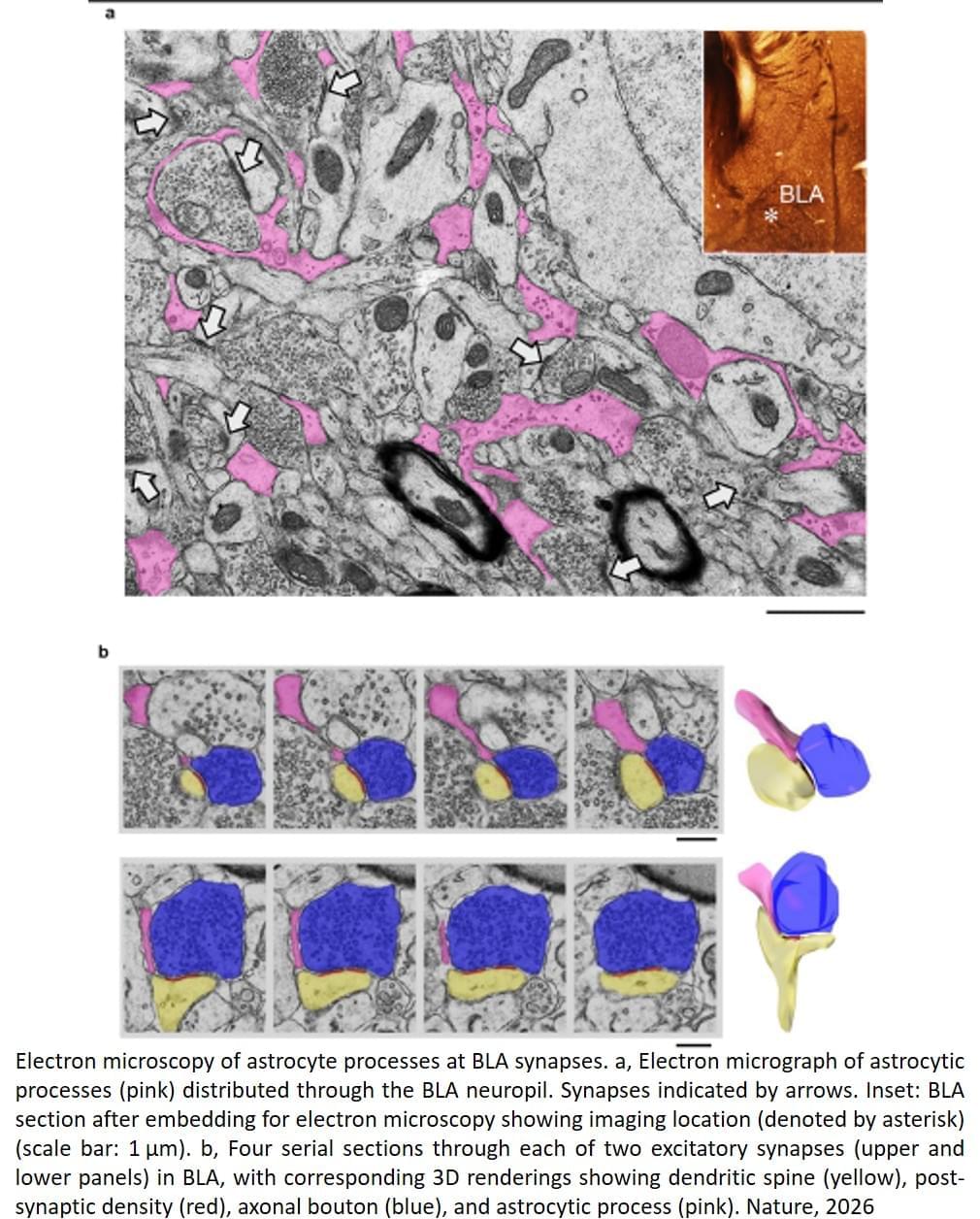
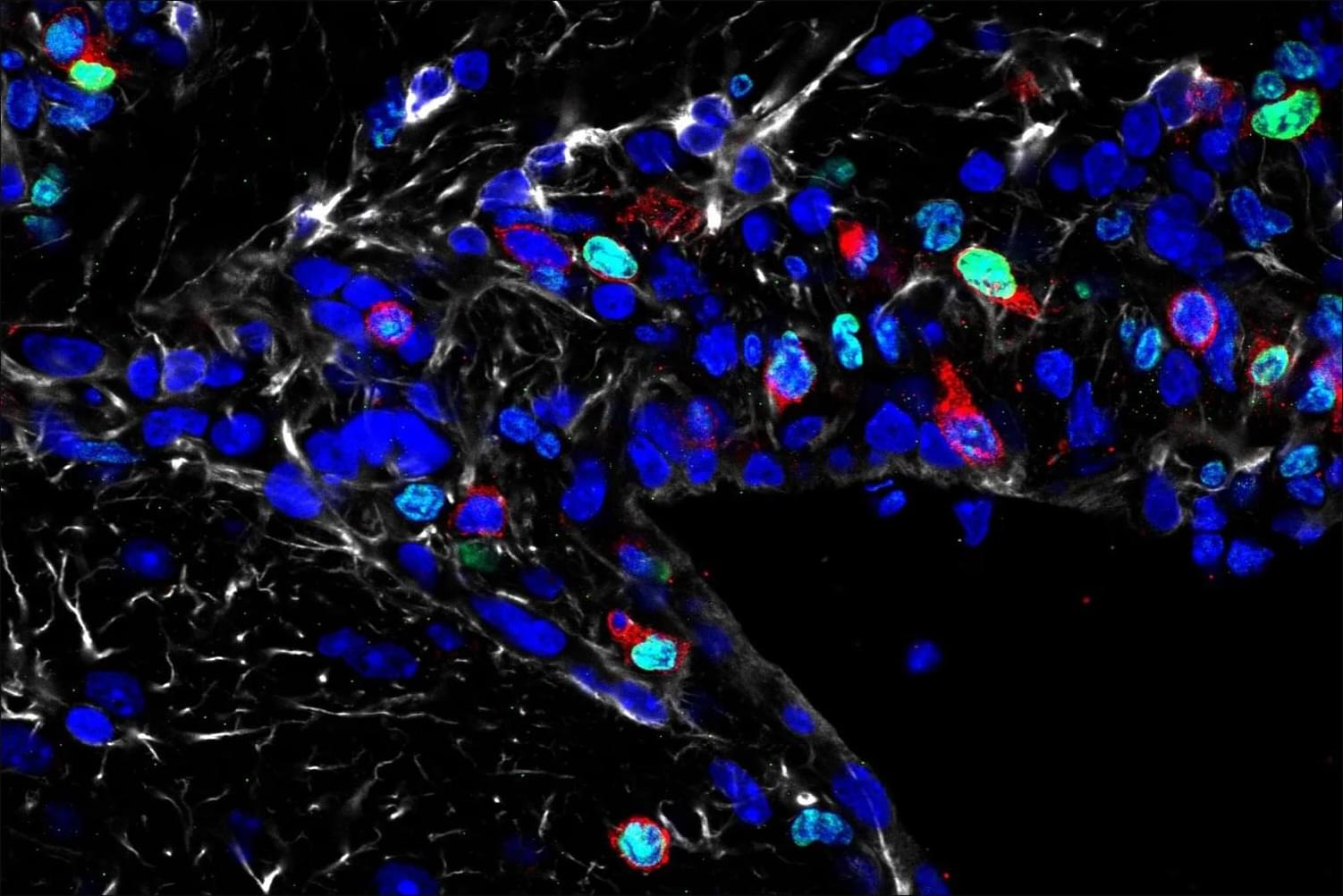
The team used a mouse model to understand how fear learning as a mechanism takes place in the brain, how fear-related memories can be retrieved, and the contribution of neurons versus astrocytes to fear learning.
Using fluorescent activity sensors, the team watched astrocytes respond in real time as fear memories were formed and later retrieved. As those memories were extinguished, astrocyte activity diminished. When the researchers then selectively increased or suppressed the signals astrocytes send to neighboring neurons, the strength of fear memories shifted in parallel, demonstrating that astrocytes are not just passive bystanders, but active participants in shaping fear.
Change in astrocyte activity also influenced neural circuits. When the astrocyte activity was disrupted, neurons could no longer form normal fear-related activity patterns and effectively transmit information about appropriate defensive reactions to brain regions that help control defensive behavior. These findings challenge neuron-centric models of fear by showing that fear memories aren’t produced by neurons alone.
The impact of disrupting astrocytes rippled beyond the amygdala. The manipulations also influenced how fear signals were relayed to the prefrontal cortex, a brain region that is key for decision-making. This suggests that astrocytes not only influence encoding of fear memories by the amygdala, but also how the brain uses those memories to determine appropriate responses to fearful situations.
Knowing that astrocytes play a key role in the retrieval of fear memories will reshape therapeutic interventions for disorders driven by persistent fearful memories such as post-traumatic stress disorder, anxiety disorders and phobias, the author said. If astrocytes help determine whether fear memories are expressed or successfully extinguished, then targeting astrocyte-related pathways, rather than neural pathways, could eventually complement neuron-focused therapies.
Picture a star-shaped cell in the brain, stretching its spindly arms out to cradle the neurons around it. That’s an astrocyte, and for a long time, scientists thought its job was caretaking the brain, gluing together neurons, and maintaining neural circuits.