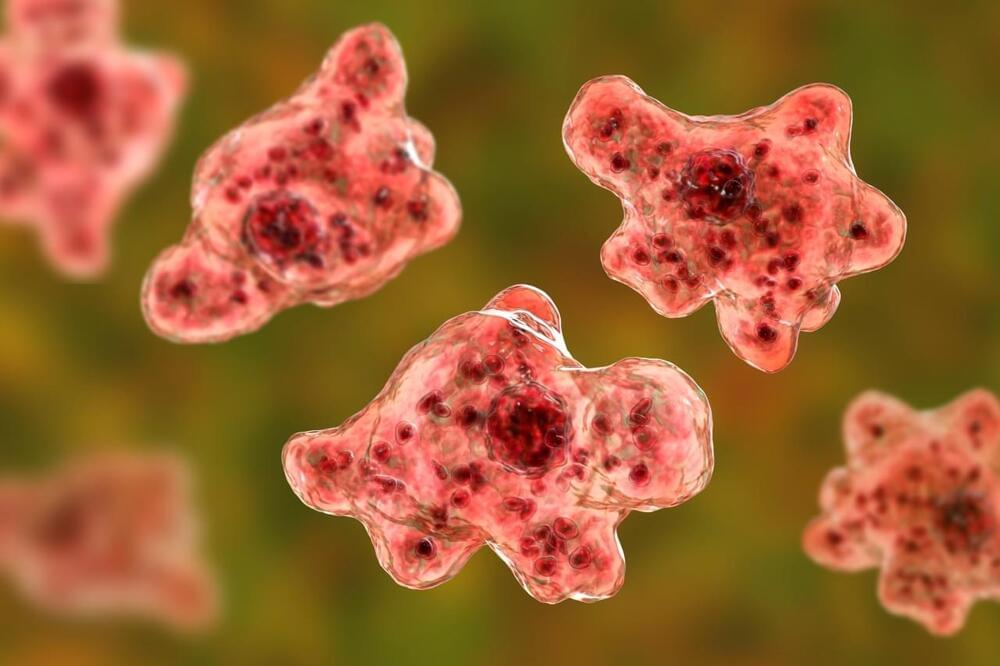
Although rare, people become infected by Naegleria fowleri when water containing the amoeba enters the body through the nose. It then travels to the brain, where it destroys brain tissue. This infection cannot spread from one person to another, and it cannot be contracted by swallowing contaminated water.
“These situations are extremely rare in the United States and in Missouri specifically, but it’s important for people to know that the infection is a possibility so they can seek medical care in a timely manner if related symptoms present,” Dr. George Turabelidze, Missouri’s state epidemiologist, said in a statement.
Symptoms can include severe headaches, fever, nausea, vomiting, stiff neck, seizures, altered mental state and hallucinations. Anyone who experiences these symptoms after swimming in a warm body of water should contact their health care provider immediately.