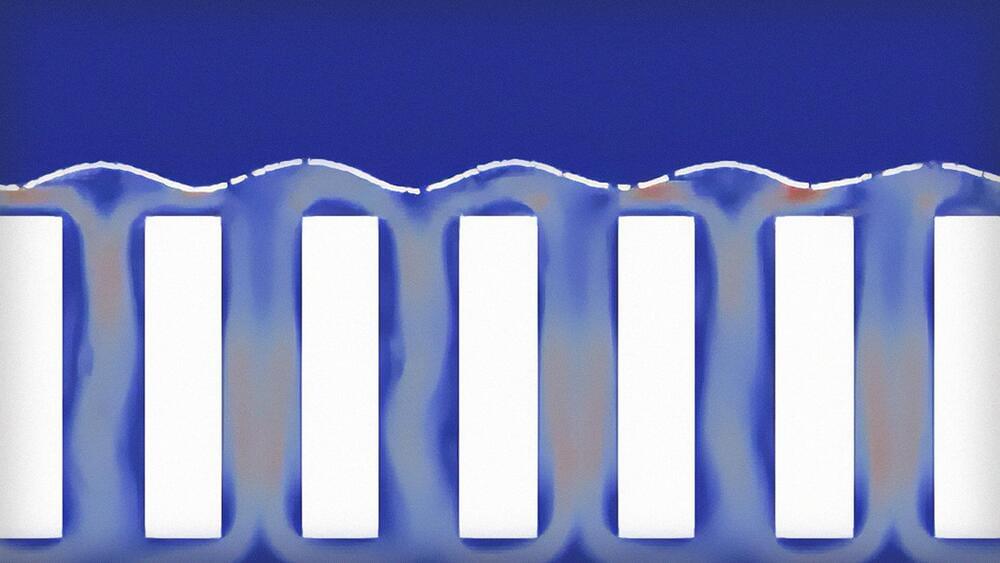
Fusion vessels have a Goldilocks problem: The plasma within needs to be hot enough to generate net power, but if it’s too hot, it can damage the vessel’s interior. Researchers at the Princeton Plasma Physics Laboratory (PPPL) are exploring ways to draw away excess heat, including several methods that use liquid metal.
One possibility, say researchers at the U.S. Department of Energy Lab, involves flowing liquid lithium up and down a series of slats in tiles lining the bottom of the vessel. The liquid metal could also help to protect the components that face the plasma against a bombardment of particles known as neutrons.
“The prevailing option for an economical commercial fusion reactor is a compact design,” said PPPL’s Egemen Kolemen, co-author of a 2022 paper on the research and an associate professor of mechanical and aerospace engineering and the Andlinger Center for Energy and the Environment. However, compactness makes handling the heat flux and neutron bombardment a bigger challenge.