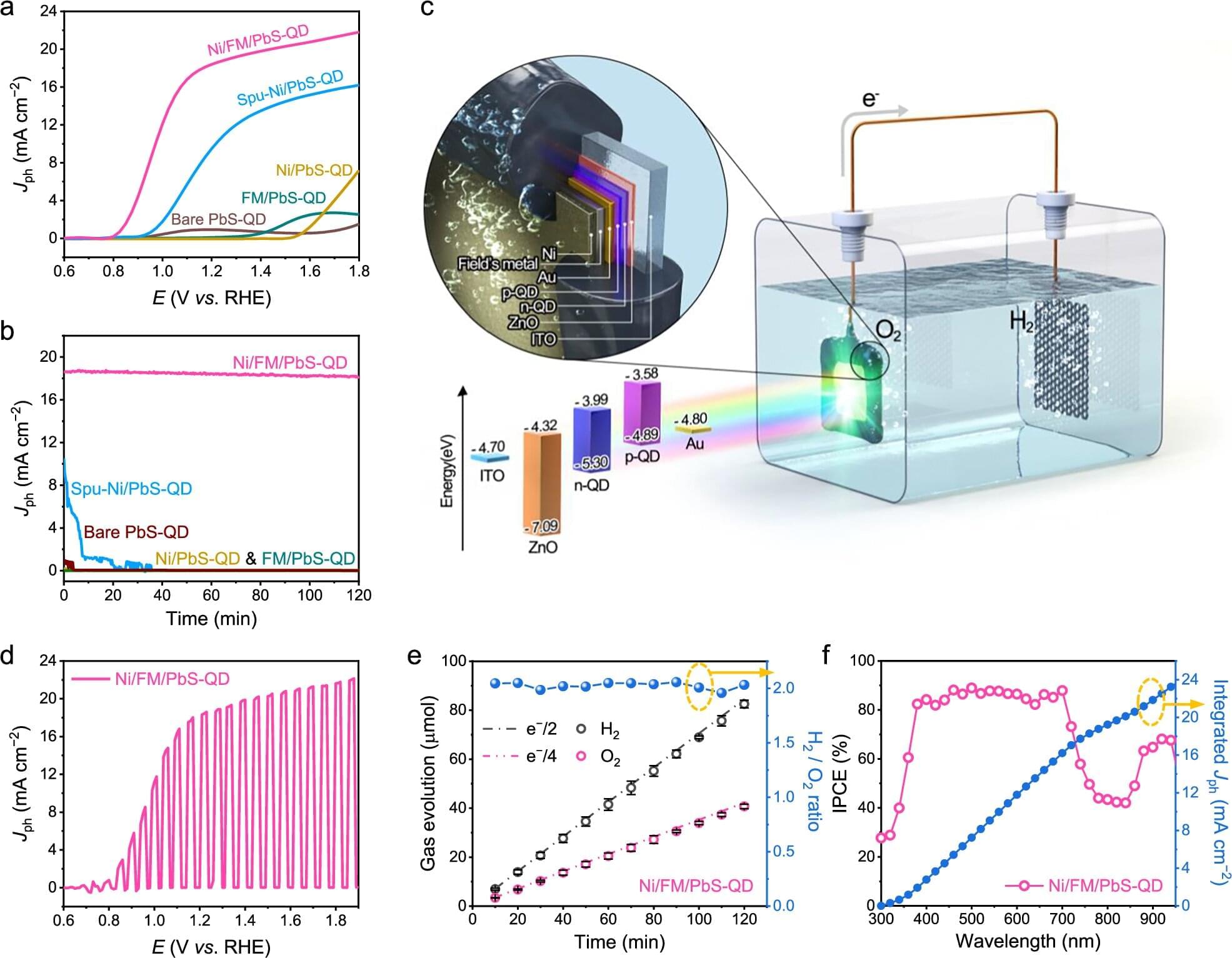
When the sun goes down, solar panels stop working. This is the fundamental hurdle of renewable energy: how to save the sun’s power for a rainy day—or a cold night. Chemists at UC Santa Barbara have developed a solution that doesn’t require bulky batteries or electrical grids. In a paper published in the journal Science, Associate Professor Grace Han and her team detail a new material that captures sunlight, stores it within chemical bonds and releases it as heat on demand.
The material, a modified organic molecule called pyrimidone, is the latest advancement in molecular solar thermal (MOST) energy storage.
“The concept is reusable and recyclable,” said Han Nguyen, a doctoral student in the Han Group and the paper’s lead author.