I think we should approach from two angles: 1. encourage and fund through government to have everyone who can to put solar on their home/building/whatever. 2. Also have the massive sites dedicated to solar and wind harvesting. Seems we could be totally solar by mid 2030s.
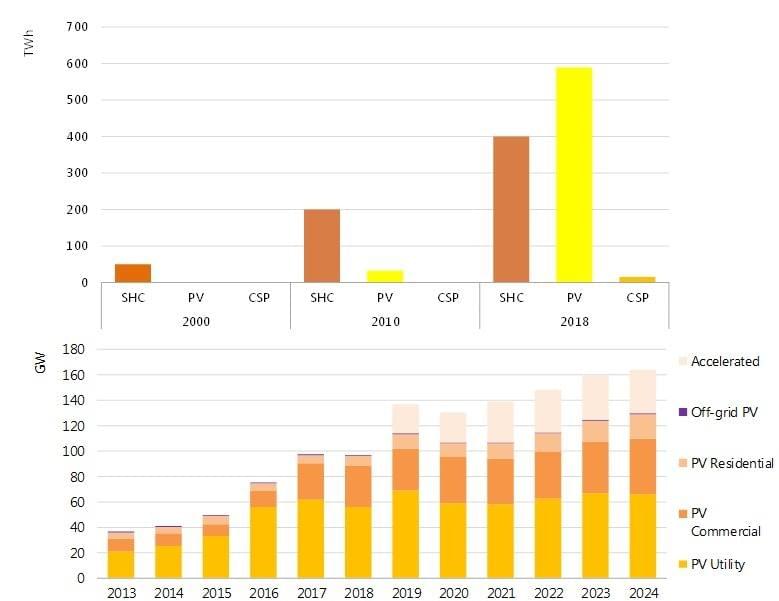
Rooftop solar panels are up to 79% cheaper than they were in 2010. These plummeting costs have made rooftop solar photovoltaics even more attractive to households and businesses who want to reduce their reliance on electricity grids while reducing their carbon footprints.
But are there enough rooftop surfaces for this technology to generate affordable, low-carbon energy for everyone who needs it? After all, it’s not just people who own their own houses and want to cut their bills who are in need of solutions like this. Around 800 million people globally go without proper access to electricity.