The work was discovered on the back of another of the artist’s paintings in a Scottish gallery.



Unlike much outdoor infrastructure, roads are not designed with any sun protection, making them prone to cracking and potentially unsafe to drive on.
Now, RMIT University engineers collaborated with Tyre Stewardship Australia (TSA) to discover the perfect blend that is both UV resistant and withstands traffic loads, with the potential to save governments millions on road maintenance annually. Their new research has found that crumb rubber, which is recycled from scrap tires, acts like sunscreen for roads and halves the rate of sun damage when mixed with bitumen.
Crumb rubber has already shown promise in making concrete stronger and more heat resistant. The new study found that the material acts so effectively as a sunscreen for roads that it actually makes the surface last twice as long as regular bitumen.

Nearly 10 years of research is giving U.S. nuclear companies the data and confidence they need to operate up to 80 years.
The amphibious assault ship USS Essex can print parts on demand, reducing the need for inventory.
One of the U.S. Navy’s largest warships is now rocking a 3D printer, allowing the crew to quickly crank out replacement parts for drones. The service hopes that additive manufacturing technology will allow it to save time and money, reducing the need to stock spare parts on hand, especially when a ship is at sea. It believes that 3D printers could someday become standard issue on every warship.

After a US Supreme Court draft decision on Roe v. Wade was leaked in May, Dr. Joshua Trebach noticed a disturbing turn in the online conversation around abortion.
“I started seeing things on social media, things like TikTok, Twitter, Facebook, Instagram, people saying ‘oh, if Roe v. Wade does get overturned, here are some secret, sneaky ways that you can drink some tea and have an abortion,’” Trebach said.
Now that Roe v. Wade has been overturned and some states are putting strict limits on abortions, there’s widespread confusion about whether the procedures are available and to whom. Physicians and poison control officials say they’re worried that people seeking abortions will turn to ineffective and dangerous methods shared online, potentially delaying or preventing safe, proven abortion care.


A Google executive said the company’s data shows TikTok and Instagram are a threat to Google Search with Gen Z, and Google is working to keep up.
Researchers at Cornell University have come up with a novel biomaterial that can be used to create artificial skin capable of mimicking the behavior of natural human tissues. Thanks to its uniqu.
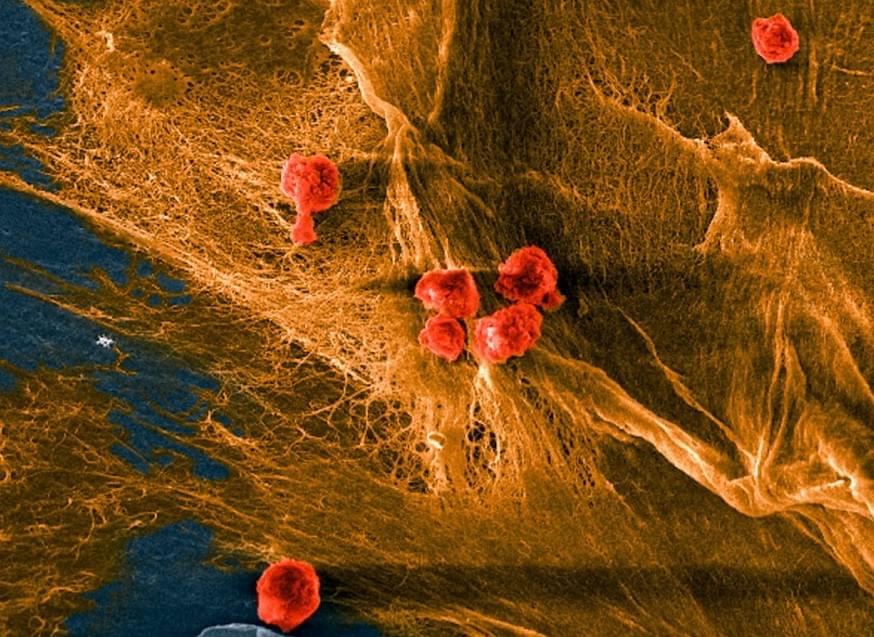
Researchers at Cornell University have come up with a novel biomaterial that can be used to create artificial skin capable of mimicking the behavior of natural human tissues.
Thanks to its unique composition, made up of collagen mixed with a ‘zwitterionic’ hydrogel, the team’s biohybrid composite is said to be soft and biocompatible, but flexible enough to withstand continued distortion. While the scientists’ R&D project remains ongoing, they say their bio-ink could one day be used as a basis for 3D printing scaffolds from patients’ cells, which effectively heal wounds in-situ.
“Ultimately, we want to create something for regenerative medicine purposes, such as a piece of scaffold that can withstand some initial loads until the tissue fully regenerates,” said Nikolaos Bouklas, one of the study’s co-lead authors. “With this material, you could 3D print a porous scaffold with cells that could eventually create the actual tissue around the scaffold.”