A better understanding of immune checkpoint signaling can help improve future checkpoint inhibitor therapeutic approaches.

Emerging trends in the disassembly of stress granules and P-bodies.
Stress granules (SGs) and P-bodies (PBs) are dynamic RNA-protein condensates regulating mRNA fate. While assembly mechanisms are well studied, emerging insights into their dissolution are now coming to light. Here, Garg et al. highlight the current understanding of disassembly mechanisms and their implications in neurodegeneration and cancer.
Discover the world of neural dust as a new frontier in biomedical technology, promising to revolutionize healthcare by enabling real-time brain monitoring an…

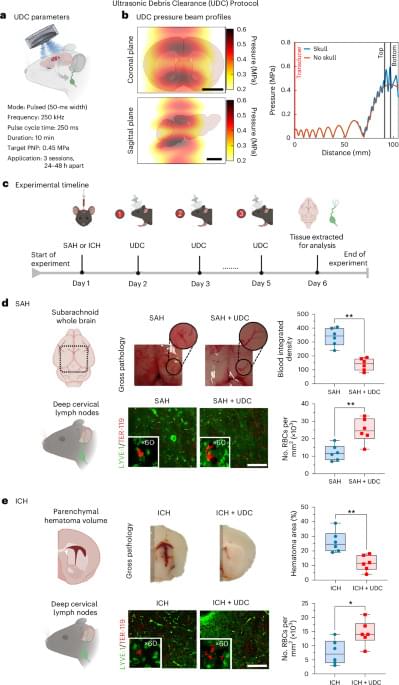

Researchers at Washington University School of Medicine in St. Louis, along with collaborators at Northwestern University, have developed a noninvasive approach to treat one of the most aggressive and deadly brain cancers. Their technology uses precisely engineered structures assembled from nano-size materials to deliver potent tumor-fighting medicine to the brain through nasal drops. The novel delivery method is less invasive than similar treatments in development and was shown in mice to effectively treat glioblastoma by boosting the brain’s immune response.
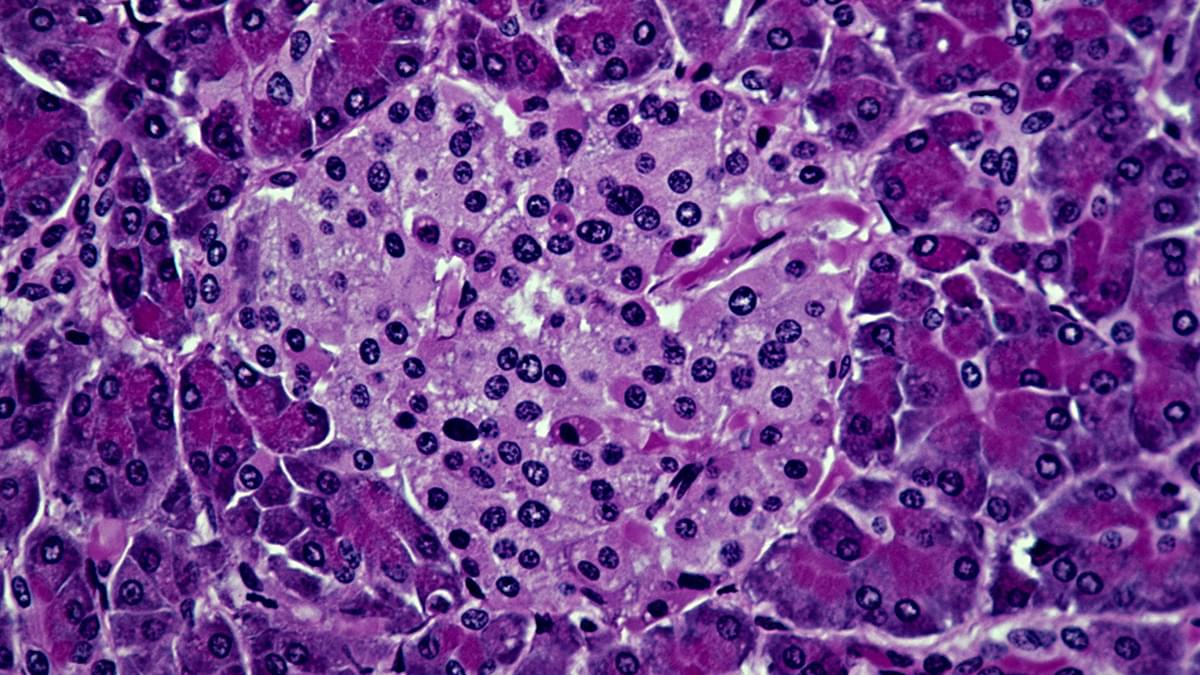
A specially devised hybrid treatment has shown tremendous potential for treating type 1 diabetes in mice, scoring full marks at preventing the condition in prediabetic animals and at reversing the condition in animals with fully developed diabetes.
What makes the new approach stand out is that it successfully combines immune system cells from both the patient mouse and a donor mouse, encouraging them to live in harmony without a need for immunosuppressive drugs for at least four months.
The researchers behind the work, led by a team from the Stanford School of Medicine, are hopeful the same approach could be successful in humans too. The treatment might also have potential for other procedures where transplants are required.
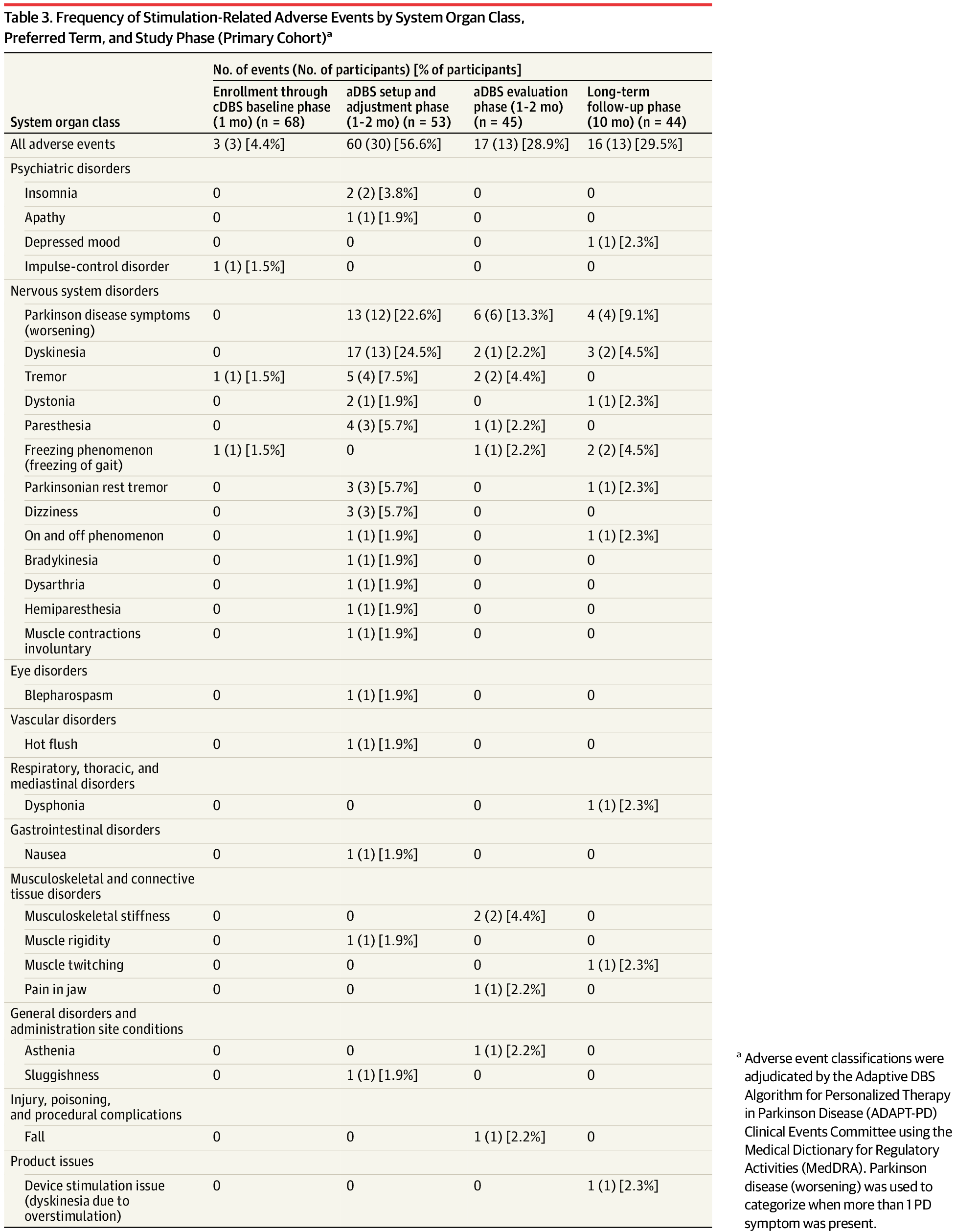
Long-term adaptive deep brain stimulation (aDBS) provided tolerable, effective, and safe therapy in persons with Parkinson disease whose symptoms were previously stable while receiving continuous DBS.
Question Is long-term adaptive deep brain stimulation (aDBS) tolerable and as effective and safe as continuous DBS (cDBS)?
Findings In this nonrandomized clinical trial with an open-label comparison between cDBS and aDBS, the primary outcome was met as the majority of participants receiving aDBS achieved a performance goal of good on-time (ie, time when symptoms were well controlled) without troublesome dyskinesia relative to stable cDBS therapy.
Meaning Long-term aDBS provided tolerable, effective, and safe therapy in persons with Parkinson disease whose symptoms were previously stable while receiving cDBS.