Discoveries by Deisseroth and his two co-recipients regarding microbial light-activated molecules led to his development of a way to manipulate selected neurons in living animals to observe changes in their behavior.



A deepening understanding of the brain has created unprecedented opportunities to alleviate the challenges posed by disability. Scientists and engineers are taking design cues from biology itself to create revolutionary technologies that restore the function of bodies affected by injury, aging, or disease — from prosthetic limbs that effortlessly navigate tricky terrain to digital nervous systems that move the body after a spinal cord injury.
With the establishment of the new K. Lisa Yang Center for Bionics, MIT is pushing forward the development and deployment of enabling technologies that communicate directly with the nervous system to mitigate a broad range of disabilities. The center’s scientists, clinicians, and engineers will work together to create, test, and disseminate bionic technologies that integrate with both the body and mind.

Cardiovascular fat deposition, found to be higher in postmenopausal women compared with premenopausal women, is a novel risk factor for cardiovascular disease. It is also believed to affect cognitive function through neuropathological pathways by changing the secretion of inflammatory cytokines and adipokines. The quality of cardiovascular fat is characterized by its radiodensity.
Summary: Greater radiodensity of perivascular adipose tissue in women during midlife was associated with decreased working memory performance later in life.
Source: NAMS
A worsening cardiovascular profile after menopause may contribute to the fact that women are disproportionately affected by dementia. A new study identified a link between cardiovascular fat volume and radiodensity and cognitive function, as well as racial differences in this association.
Study results will be presented during The North American Menopause Society (NAMS) Annual Meeting in Washington, DC, September 22–25, 2021.
I believe if superintelligence can be digitized into computer code then essentially a microchip could send electrical impulses to one’s brain noninvasive like the microchip that heals from Ohio state and then superintelligence could be attained and the biological wetware could be easily acquire the biological singularity. Much like the moto that Apple has all things digital a new superintelligence attribute could uploaded and the human could evolve or gene edit from a smartphone also the impulse could be non invasive like low level electrical impulse sending data to the brain using existing hardware. We could be as advanced as any exterrestial civilization in a couple keystrokes using existing hardware.
Popular expectations for the future are helplessly colored by present trends. The assumption is always that whatever’s going on now can be safely extrapolated into the future along a linear (or, per Kurzweil, logarithmic) curve. So it was that during the space race, baby boomers took for granted that we’d have fully colonized the solar system by the year 2000.

Summary: Mouse study reveals chronic stress affects neurogenesis in the dentate gyrus.
Source: Tokyo University of Science.
Depression is a serious medical condition that plagues modern society. Several theories have been proposed to explain the physiological basis of depression, of which the “neurogenic hypothesis of depression” has garnered much attention.
To use the metaphor of our Information Age, consciousness to humans is as Cloud to computers. Just like your smartphone, your brain is a ‘bio’-logical computing device of your mind, an interface for physical reality. Our minds are connected into the greater mind-network, as computers in the Cloud. Viewed in this way, consciousness is ‘non-local’ Cloud, our brain-mind systems are receivers, processors and transmitters of information within that Cloud. What were the most significant factors in evolution of the human mind? What’s the connection between quantum physics and consciousness? What role does quantum information play in our self-reflective consciousness? What is non-local consciousness? Do our minds create reality? These are some of the most salient questions addressed in this Part II of the documentary.
#consciousness #evolution #mind #documentary #film
By Elizabeth Titovskaya.
“Information is a difference that makes a difference.” ―Gregory Bateson.
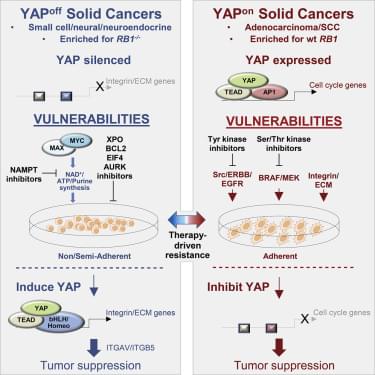
Cancer heterogeneity impacts therapeutic response, driving efforts to discover over-arching rules that supersede variability. Here, we define pan-cancer binary classes based on distinct expression of YAP and YAP-responsive adhesion regulators. Combining informatics with in vivo and in vitro gain-and loss-of-function studies across multiple murine and human tumor types, we show that opposite pro-or anti-cancer YAP activity functionally defines binary YAPon or YAPoff cancer classes that express or silence YAP, respectively. YAPoff solid cancers are neural/neuroendocrine and frequently RB1−/−, such as retinoblastoma, small cell lung cancer, and neuroendocrine prostate cancer. YAP silencing is intrinsic to the cell of origin, or acquired with lineage switching and drug resistance. The binary cancer groups exhibit distinct YAP-dependent adhesive behavior and pharmaceutical vulnerabilities, underscoring clinical relevance. Mechanistically, distinct YAP/TEAD enhancers in YAPoff or YAPon cancers deploy anti-cancer integrin or pro-cancer proliferative programs, respectively. YAP is thus pivotal across cancer, but in opposite ways, with therapeutic implications.
Pearson et al. demonstrate that YAP/TAZ, well-known oncogenes, are tumor suppressors in a large group of cancers. Pan-cancer analyses reveal that opposite YAP/TAZ expression, adhesive behavior, and oncogenic versus tumor suppressor YAP/TAZ activity functionally stratify binary cancer classes, which interchange to drive drug resistance. Contrasting YAPoff/YAPon classes exhibit unique vulnerabilities, facilitating therapeutic selection.
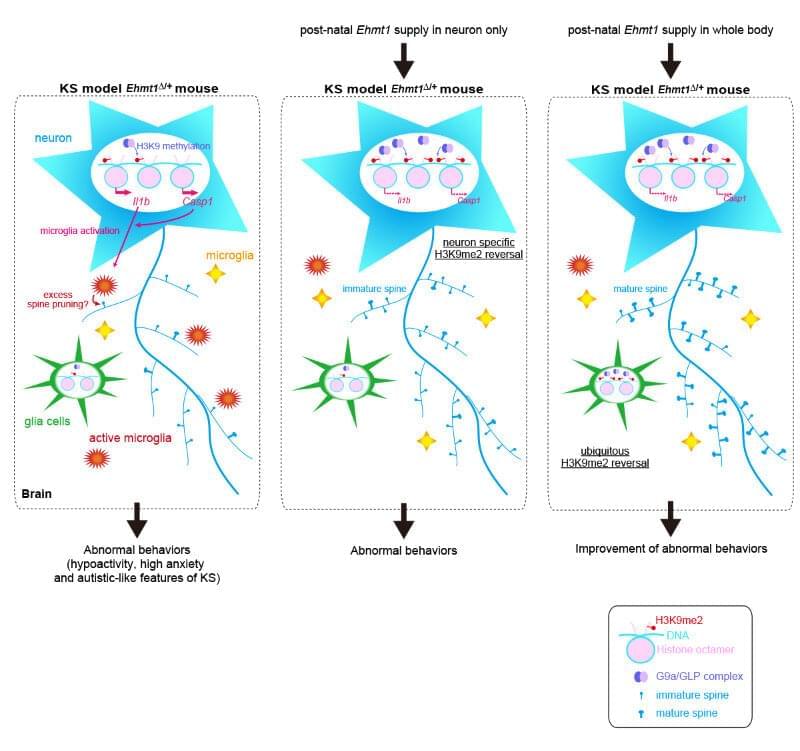
As this is the first report of neuro-inflammation in Kleefstra syndrome, the next step is to find out if it also occurs in the human condition. Shinkai believes the chances are high and says he would not be surprised if other neurological diseases caused by epigenetic dysregulation were also related to abnormal inflammation in the brain.
Researchers at the RIKEN Cluster for Pioneering Research (CPR) in Japan report that Kleefstra syndrome, a genetic disorder that leads to intellectual disability, can be reversed after birth in a mouse model of the disease. Published in the scientific journal iScience, the series of experiments led by Yoichi Shinkai showed that postnatal treatment resulted in improved symptoms, both in the brain and in behavior.
Normally, we get two good copies of most genes, one from each parent. In Kleefstra syndrome, one copy of the EHMT1 gene is mutated or missing. This leads to half the normal amount of GLP, a protein whose job is to control genes related to brain development through a process called H3K9 methylation. Without enough GLP, H3K9 methylation is also reduced, and the connections between neurons in the brain do not develop normally. The result is intellectual disability and autistic-like symptoms. “We still don’t know if Kleefstra syndrome is a curable disease after birth or how this epigenetic dysregulation leads to the neurological disorder,” says Shinkai. “Our studies in mice have provided new information about what causes the behavioral abnormalities associated with the syndrome and have shown that a cure is a real possibility in the future.”
Reasoning that extra GLP might be an effective treatment, the researchers performed a series of experiments in mice that were engineered to have only one good copy of the EHMT1 gene. The brains of these mice show characteristics of the human condition, including 40% less GLP and 30% less H3K9 methylation. The mice also display several behaviors seen in humans with Kleefstra syndrome, such as reduced locomotion and greater anxiety. After each experiment, the researchers measured these factors and compared them to normal mice to see if the treatment had been effective.