Earth, Mars and Venus all looked pretty similar when they first formed. Today, Mars is dry, cold, and dusty; Venus has a hot, crushing atmosphere. Why did these sibling planets turn out so different?

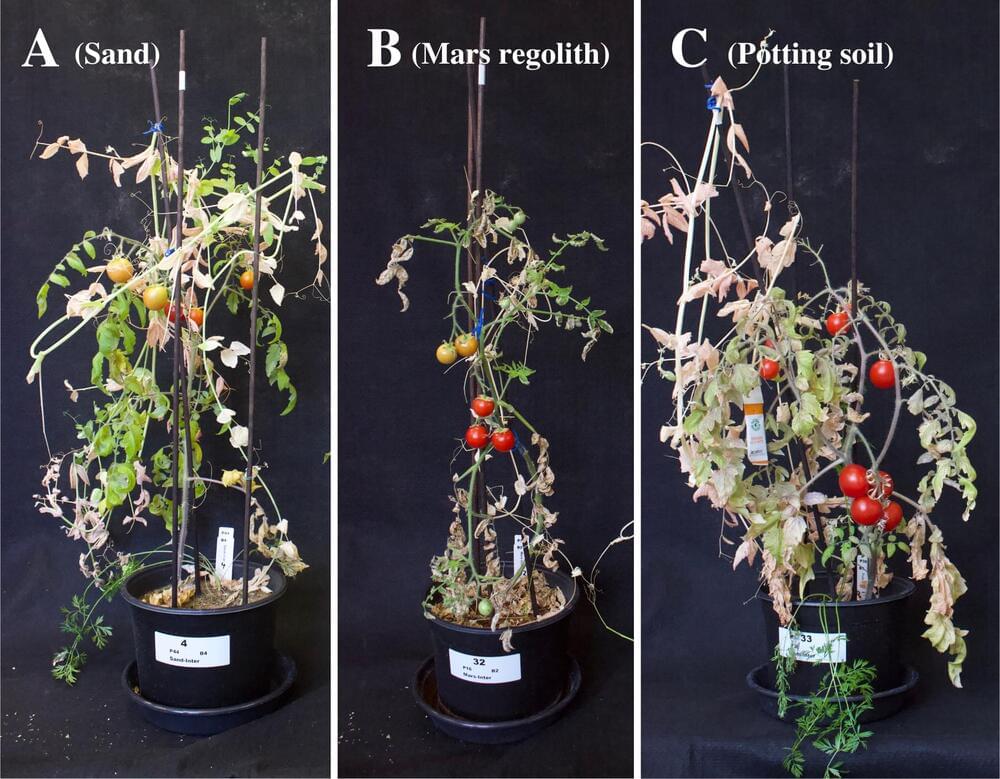
If humans build settlements on Mars, how will they feed ourselves? Waiting on deliveries from Earth would take too long and costs would be exorbitant, since getting to the Red Planet is currently a nine-month one-way journey. On top of that, dehydrating foodstuff—the best preservation method for perishables sent to space—removes vital nutrients.
More than likely, Martian settlers will need to grow their own food.
Researchers are now exploring how best to optimize crop yield on Mars using intercropping, a technique perfected by Maya farmers centuries ago that involves growing multiple plants in close proximity to one another. Their findings—published this month in the journal Plos One—could not only benefit the pioneers who end up colonizing the Red Planet, but also farmers here on Earth amid a rapidly changing climate.

A team of scientists, astrophysicists and physicists, in an experiment called BICEP2 (Background Imaging of Cosmic Extragalactic Polarisation 2), carried out over nine years at an astronomical observatory at the South Pole, reported that they had discovered undeniable traces of a much sought-after phenomenon in astrophysics: gravitational waves. It was also announced that the method used to make the discovery had provided an important confirmation of the theoretical model of Big Bang cosmology, and would allow the first moments after this primordial explosion—the moment of creation for modern astrophysics—to be studied experimentally.
When you don’t find gravitational waves…
If we imagine space and time as the surface of an ocean, gravitational waves can be thought of as ripples in that ocean. More precisely, gravitational waves are theoretical ripples in space-time, first predicted by Albert Einstein in 1916 on the basis of his general theory of relativity. Like electromagnetic waves, which are produced by the oscillation of an electric charge, it is thought that a sufficiently strong oscillation of a very massive object should produce gravitational waves, which carry energy in the form of gravitational energy.




Often perceived as abstract and challenging, physics covers fundamental aspects of the universe, from the tiny world of quantum mechanics to the vast cosmos of general relativity. However, it often comes with intricate mathematical formulations that intimidate many learners. Visual Intuitive Physics is an emerging field that seeks to transform this complexity into accessible visual experiences, making physics more tangible and relatable. By employing visual aids and intuitive methodologies, this approach enhances the understanding of physical principles for students, researchers, and enthusiasts.
Understanding complex physics concepts often requires intuitive visualization that transcends verbal and mathematical explanations. Visualization in physics involves using graphs, diagrams, simulations, and other visual tools to provide a tangible understanding of abstract concepts. For instance, Marr and Bruce emphasized that visual tools significantly enhance conceptual understanding in students by providing concrete ways to comprehend physical laws.
Visualization helps bridge the gap between theoretical concepts and practical understanding. Per Kozma and Russell, visualization is pivotal in building cognitive structures that make understanding and remembering scientific principles easier. This is particularly significant for concepts that lack direct physical analogs, such as quantum mechanics and relativity.
NYU Abu Dhabi researchers have unveiled a novel 2D material improving optical modulation for advanced systems and communications.
Responding to the increasing demand for efficient, tunable optical materials capable of precise light modulation to create greater bandwidth in communication networks and advanced optical systems, a team of researchers at NYU Abu Dhabi’s Photonics Research Lab (PRL) has developed a novel, two-dimensional (2D) material capable of manipulating light with exceptional precision and minimal loss.
Tunable optical materials (TOMs) are revolutionizing modern optoelectronics, electronic devices that detect, generate, and control light. In integrated photonics circuits, precise control over the optical properties of materials is crucial for unlocking groundbreaking and diverse applications in light manipulation. Two-dimensional materials like Transition Metal Dichalcogenides (TMDs) and graphene exhibit remarkable optical responses to external stimuli. However, achieving distinctive modulation across a short-wave infrared (SWIR) region while maintaining precise phase control at low signal loss within a compact footprint has been a persistent challenge.

If you’re still rinsing your teeth with water after brushing them, it may be time to stop. There are actually some benefits to leaving that extra bit of toothpaste on your teeth. I used to rinse after brushing my teeth — and even went back over them with a wet toothbrush — to remove any remnants of toothpaste left in my mouth. But then I found out from a dentist’s TikTok video that doing that isn’t the most effective method of keeping your chompers in good shape.
Instead, I now spit out as much toothpaste as I can without swishing with water, so I can allow the toothpaste to do its job just a little longer. However, I wasn’t sure why I was doing this — or what the benefits were — until I spoke with an expert.
I talked to Dr. Edmond Hewlett, consumer advisor for the American Dental Association and a professor at UCLA School of Dentistry, to find out why you shouldn’t rinse your mouth with water after brushing your teeth. Here’s the answer. For more tips, here’s why you should floss before brushing your teeth.