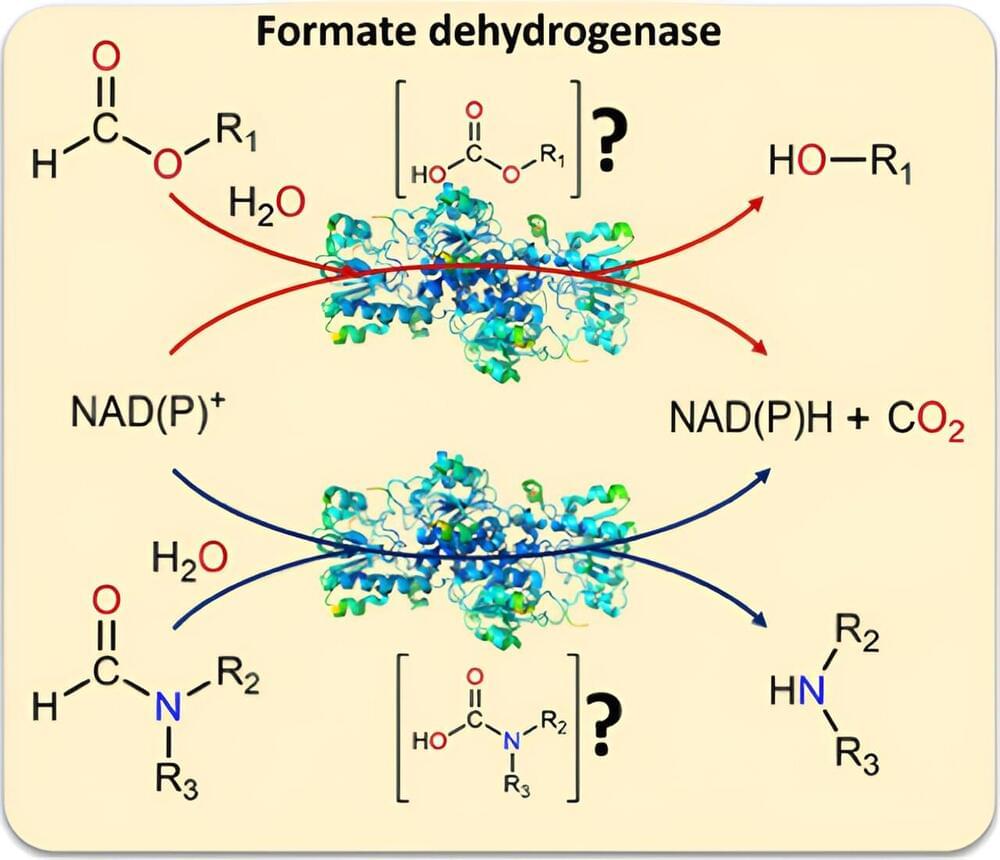
A large number of applications in the chemical industry rely on the molecules NADH or NADPH as fuel. A team led by Professor Dirk Tischler, head of the Microbial Biotechnology working group at Ruhr University Bochum, used a biocatalyst to study their production in detail.
The researchers proved that, in addition to formate, the biocatalyst formate dehydrogenase can also convert formamides. This means, for one thing, that the enzyme can also cleave the difficult-to-break C–N bond. For another, formamides are a common solvent.
“This opens up completely new possibilities for poorly soluble NADH reactions as well as NADPH-dependent reactions,” says Tischler.