Super cool paper where Jeppesen et al. discover and characterize a new type of large extracellular vesicle (EV) that they call blebbisomes! These blebbisomes have active mitochondria as well as other organelles (except nucleus), secrete and take up smaller EVs, and can reach sizes of up to 20 micrometers! #cellbiology #molecularbiology #biochemistry
Cells release a variety of 30-to 10,000-nm lipid-bilayer-enclosed extracellular vesicles (EVs) to facilitate cell-to-cell and cell-to-environment communication by packaging signalling molecules to avoid degradation1,2,3,4,5 and escape immune surveillance6,7,8,9. EVs may interact with target cells through contact between molecules on the EV surface with receptors on the cell surface to relay signals. In addition, modulation of recipient cell behavior may follow uptake of EVs cargo, including bioactive proteins, lipids and nucleic acids. EVs have emerged as important actors and agents of intercellular communication in normal cell biology and pathological conditions2,4,6.
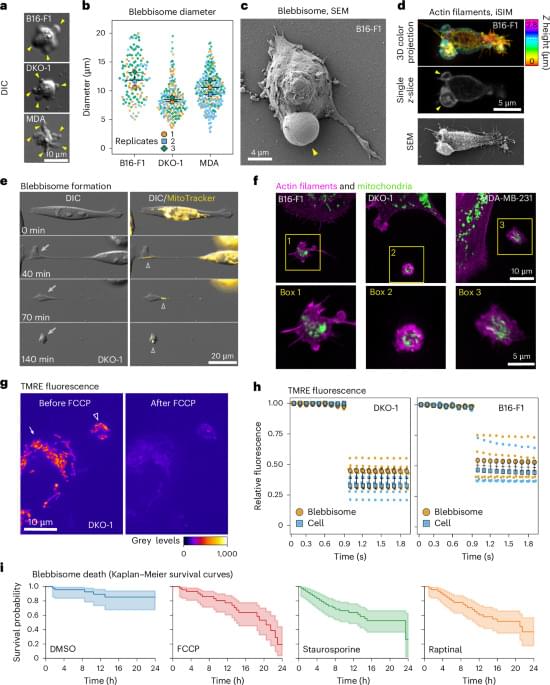
Here, we identify blebbisomes, an exceptionally large functional EVs, that are actively released by human and mouse cells, remain motile independently of cells and have the capacity to both take up EVs and secrete exosomes and microvesicles. Blebbisomes are the largest type of EV described so far with an average diameter of 10 µm but can be as large as 20 µm, with an area commonly larger than 50 µm2. After being released from motile cells, blebbisomes display marked contractility-dependent ‘blebbing’ behaviour. Both normal and cancer cells release blebbisomes that contain active, healthy, mitochondria further distinguishing them from other large EVs (lEVs) such as exophers10,11 and migrasomes12 that function in the removal of damaged mitochondria from cells under stress conditions. In addition, blebbisomes contain many other cellular organelles including endoplasmic reticulum (ER), Golgi apparatus, ribosomes, lysosomes, endosomes, multivesicular endosomes (MVEs) and autophagosomes/amphisomes, as well as cytoskeletal elements; however, they lack a definable nucleus.